Antibody drugs exert antitumor effects by regulating immune function. Their core mechanism lies in specific binding to antigens on the surface of tumor cells, enabling precise killing of tumor cells in synergy with immune cells. In this process, the Fc region of antibodies mainly relies on three key effector functions—ADCC, ADCP, and CDC—which together form the antibody-mediated antitumor immune network.
This article introduces GemPharmatech’s in vitro immune-related cytotoxicity evaluation platform and illustrates its capabilities through a series of case studies that show how each mechanism is measured, compared, and applied in drug development.
Mechanisms:
ADCC (Antibody-Dependent Cell-mediated Cytotoxicity)
When the Fab segment of an antibody binds to antigenic epitopes on tumor cells or virus-infected cells, its Fc segment specifically interacts with Fc receptors (FcR) on the surface of killer cells such as NK cells and macrophages. This "bridging" effect activates the killer cells, directly initiating the cytotoxic program against target cells.
ADCP (Antibody-Dependent Cellular Phagocytosis)
After antibodies bind to antigens on tumor cell surfaces, their Fc segments can bind to Fcγ receptors (e.g., FcγRIII/CD16A, FcγRII/CD32A, FcγRI/CD64) on effector cells like macrophages. This triggers phagocytic signals in macrophages, significantly enhancing their ability to phagocytose and eliminate tumor cells.
CDC (Complement Dependent Cytotoxicity)
The formation of immune complexes by antibodies binding to tumor cell surface antigens recruits complement protein C1q and activates the classical pathway of the complement system. Through a series of cascade reactions, the membrane attack complex (MAC) is ultimately formed. This complex directly inserts into the tumor cell membrane, leading to cell lysis and apoptosis induction for effective tumor cell killing.
These mechanisms synergize to link the targeting of antibodies with the effector functions of the immune system, serving as the core foundation for the antitumor activity of antibody drugs.
Case Study 1: ADCC Activity Detection of Rituximab
Objective: Raji is a human lymphoma cell line that endogenously expresses CD20. Peripheral Blood Mononuclear Cells (PBMC) were used as effector cells to detect the ADCC effect of Rituximab, a monoclonal antibody targeting CD20, on tumor cells.
Result: Rituximab specifically targets and kills CD20-expressing Raji tumor cells, with its cytotoxic capacity showing a significant dose-dependent enhancement.
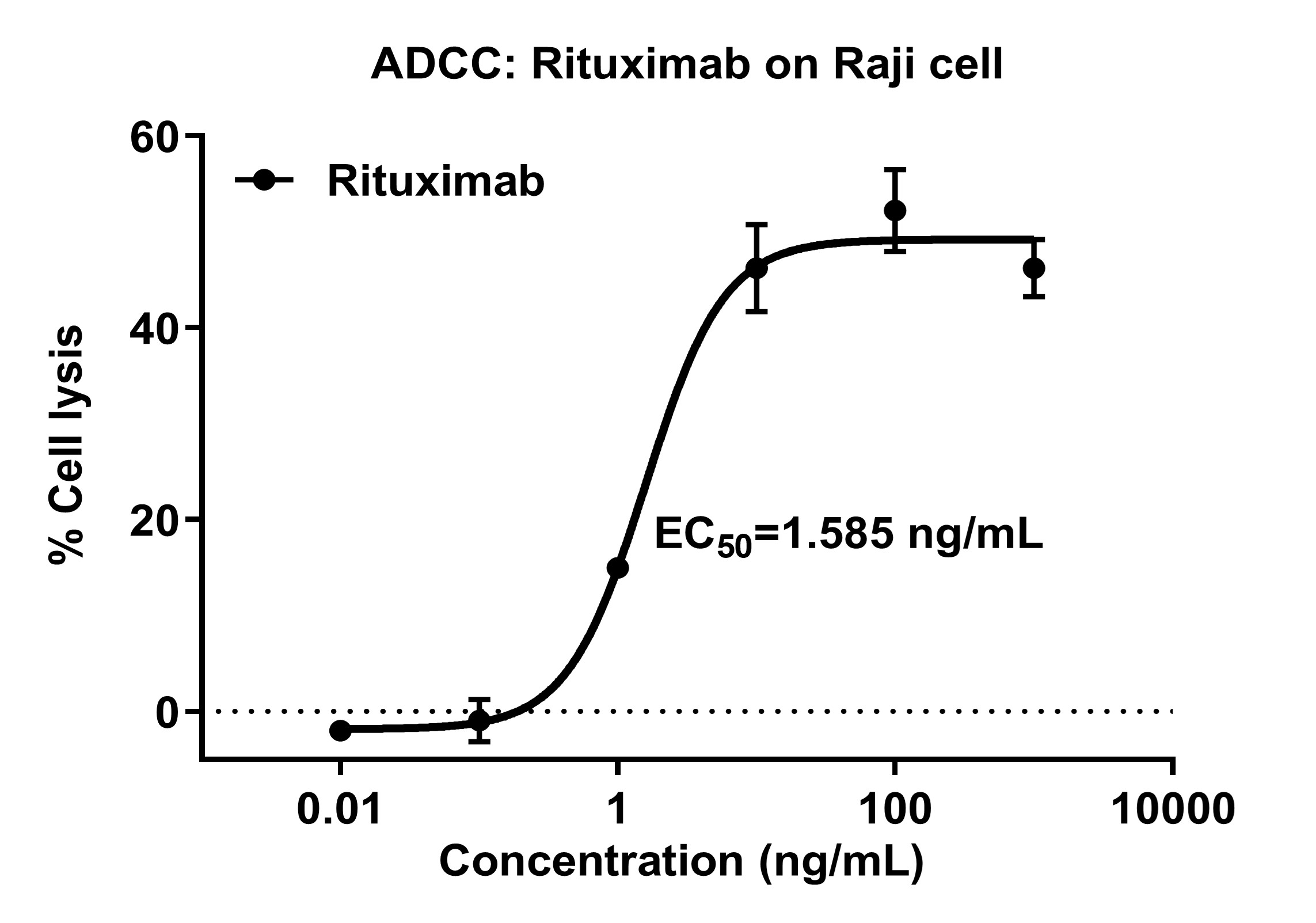
Figure 1: ADCC Activity Assay of Rituximab
Dose-response curve of Rituximab-induced ADCC against CD20⁺ Raji cells (PBMC as effector cells). % Cell lysis is plotted against Rituximab concentration (ng/mL, log scale), showing dose-dependent cytotoxicity with EC₅₀ = 1.585 ng/mL. Error bars denote variability.
Case Study 2: ADCP Activity Assay of Rituximab
Objective: In vitro-induced monocyte-derived macrophages were used to evaluate the ADCP effect of Rituximab on Raji cells via flow cytometry.
Result: Rituximab can effectively activate the ADCP effect of monocyte-derived macrophages, significantly enhancing their phagocytic capacity against Raji cells.
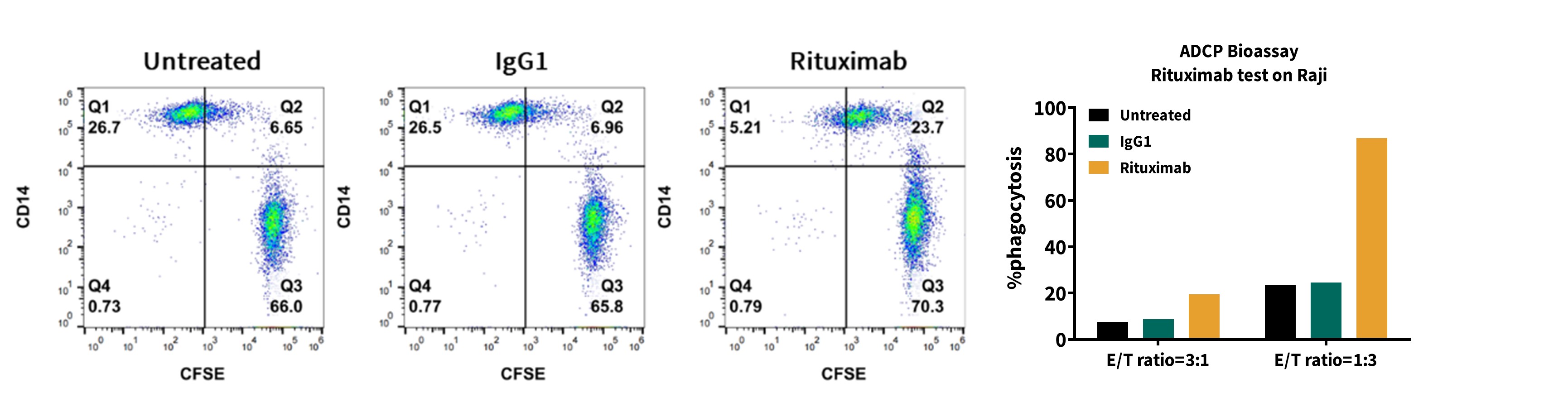
Figure 2: ADCP Activity Assay of Rituximab
Flow cytometry plots (left, CD14 vs CFSE) and phagocytosis bar graph (right) showing Rituximab’s effect on monocyte-derived macrophages’ phagocytosis of Raji cells. Untreated, IgG1, and Rituximab groups are compared, with Rituximab significantly enhancing ADCP at two effector-to-target (E:T) ratios.
Case Study 3: CDC Activity Assay of Rituximab
Objective: To evaluate the efficacy of Rituximab in killing Raji cells through the CDC pathway.
Result: Rituximab can kill Raji cells via CDC, with the effect being concentration dependent.
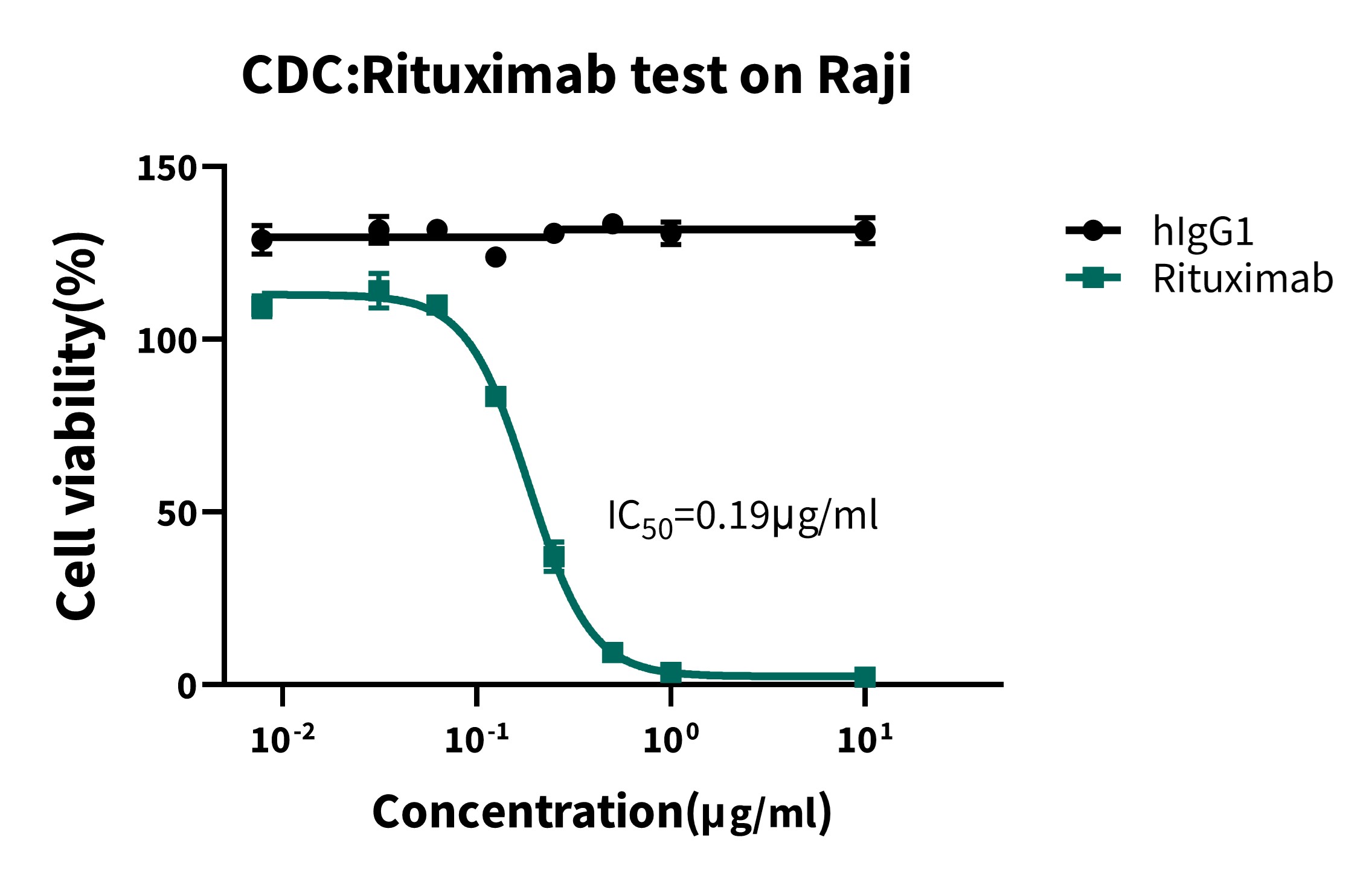
Figure 3: CDC Activity Assay of Rituximab
Dose-response curve of Rituximab-induced complement-dependent cytotoxicity (CDC) against Raji cells, compared to hIgG1. Cell viability (%) is plotted against Rituximab concentration (μg/mL, log scale), showing concentration-dependent cytotoxicity with IC₅₀ = 0.19 μg/mL.
Case Study 4: Evaluation of ADCC Effects of Human- and Mouse-Derived NK Cells
Objective: Human NK cells isolated from PBMCs and mouse NK cells isolated from the spleen of huHSC-NCG-hIL2 mice were used as effector cells. The ADCC capacities of Trastuzumab and Margetuximab against human breast cancer JIMT-1 cells were evaluated, comparing the application effects of human- and mouse-derived NK cells in drug evaluation.
Result: NK cells derived from huHSC-NCG-hIL2 mice can stably reproduce the dose-dependent effect of the ADCC drug Trastuzumab in vitro, with data trends highly consistent with human NK cells isolated from PBMCs (human PBNK). Meanwhile, they can accurately distinguish the pharmacodynamic differences between Trastuzumab and its ADCC-enhanced modified drug Margetuximab.
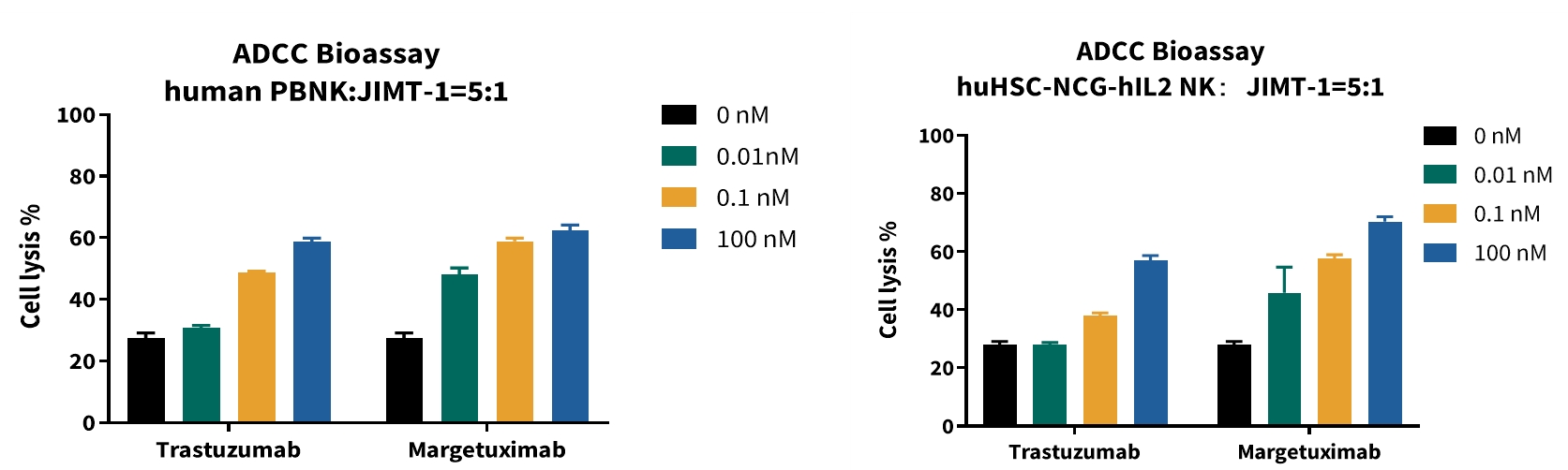
Figure 4: ADCC Effects of Human- and Mouse-Derived NK Cells
Bar graphs showing ADCC activity of Trastuzumab and Margetuximab against JIMT-1 cells, using human PBNK (left) and mouse huHSC-NCG-hIL2 NK cells (right) as effectors (E:T = 5:1). Cell lysis (%) is plotted against drug concentration, demonstrating dose-dependent activity and consistent pharmacodynamic differentiation between the two antibodies.
Case Study 5: Detection of Rituximab’s ADCC and ADCP Activities by Reporter Gene Assay
Objective: Engineered Jurkat-NFAT-CD16a and Jurkat-NFAT-CD32a reporter gene cell lines were used as effector cells to verify the ADCC and ADCP activities of Rituximab.
Result: Only in the experimental group with tumor cells present, Rituximab could activate the NFAT pathway, demonstrating ADCC activity.
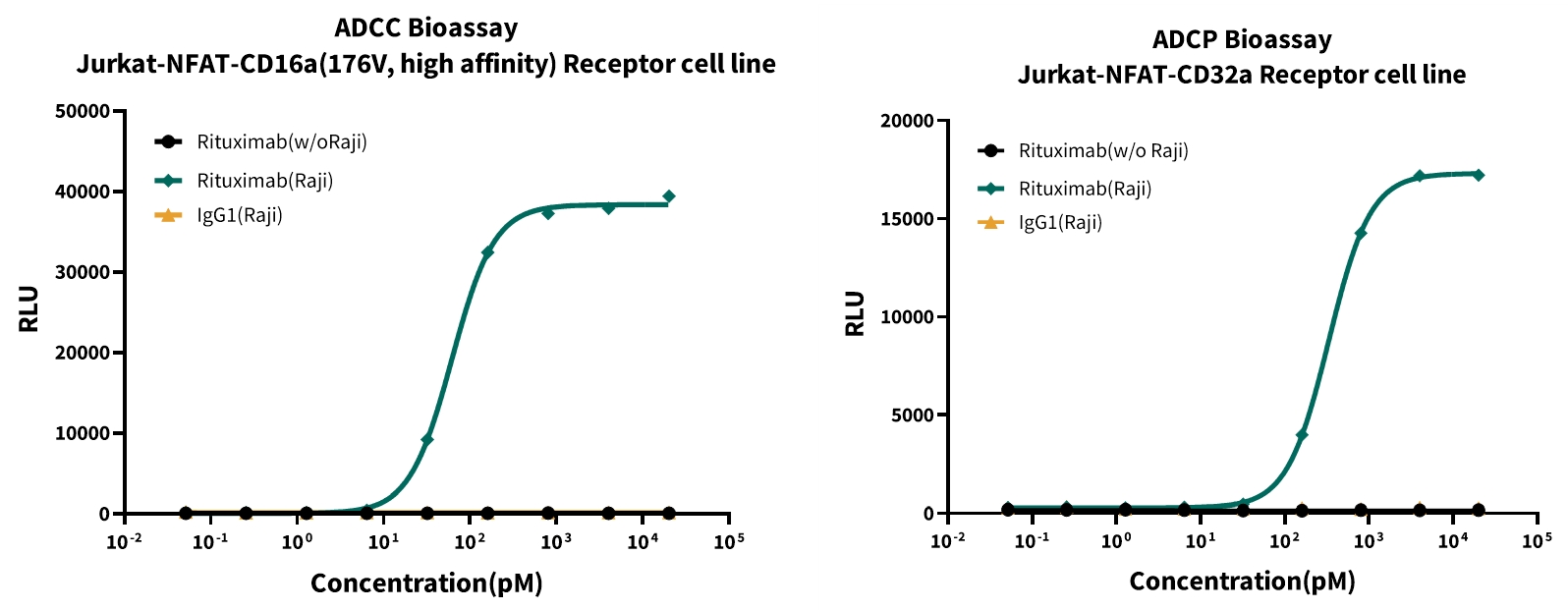
Figure 5: Detection of Rituximab’s ADCC and ADCP Activities by Reporter Gene Assay
Left: ADCC bioassay with Jurkat-NFAT-CD16a reporter cells, showing Rituximab-induced NFAT activation (RLU) against Raji cells in a concentration-dependent manner (pM). Right: ADCP bioassay with Jurkat-NFAT-CD32a reporter cells, demonstrating Rituximab’s concentration-dependent activation of ADCP against Raji cells, compared to IgG control.
GemPharmatech Advancing Drug Discovery
Establishing a comprehensive immune-related cytotoxicity evaluation platform is part of GemPharmatech’s overall effort to advance drug discovery. This platform enables systematic assessment of ADCC, ADCP, and CDC effects, providing robust technical support for the screening and optimization of candidate drugs and improving the success rate of preclinical research.
Interested in learning more about our efficacy services or models? Contact us directly to learn how GemPharmatech enables research and accelerates drug discovery.


