1. Project Design:
Based on project requirements, we provide services including project design, material preparation, antigen design, screening, and validation plan design.
2. Animal Immunization:
According to target needs, we offer various immunization methods, including small molecules, DNA, mRNA, proteins, and peptides. We also cover different types of targets, such as single-pass transmembrane, multiple-pass transmembrane, and cytokine types.
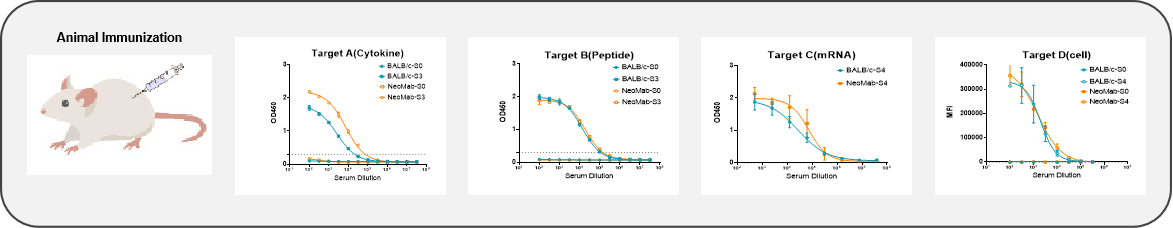
The immune response of NeoMab® mice to different antigens is comparable to or even superior to that of BALB/c mice
3. Molecular Discovery and Screening:
Based on target requirements, we offer different forms of fully humanized antibodies, including standard fully humanized antibodies, single-chain "nanobodies," and common light chain "bispecific antibodies" to meet antibody discovery needs. We provide screening platforms such as hybridoma fusion, display technology, and single B cell technology, allowing for the rapid acquisition of target molecules.
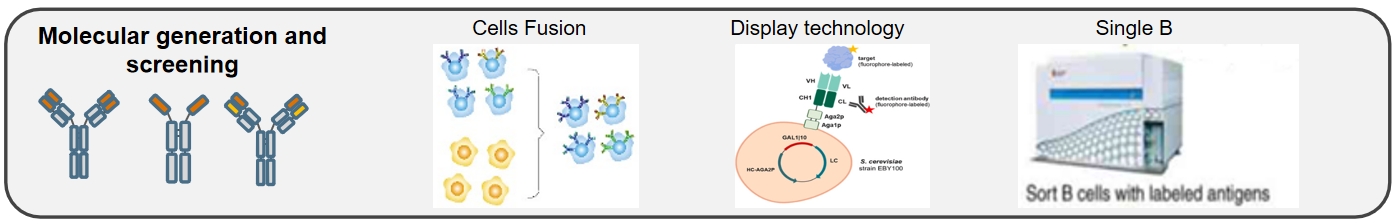
■ Cell Fusion
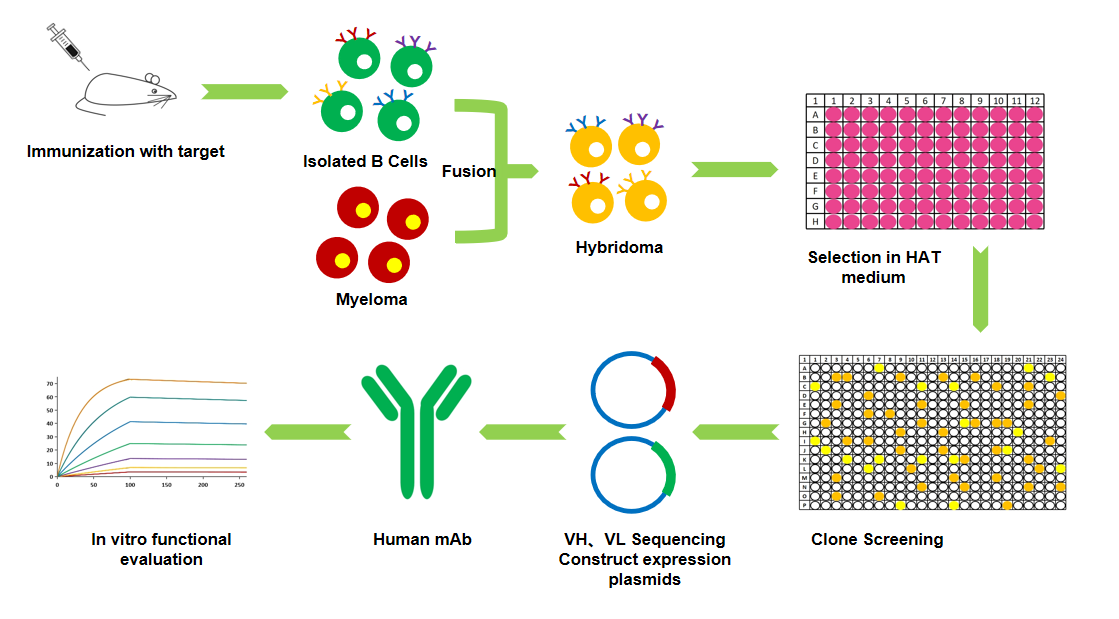
Our Advantages:
A diverse range of mature immunization protocols to meet the personalized needs for different targets and immune responses.
Stable and efficient electrofusion technology ensures the highest probability of obtaining the desired hybridoma cells.
High-throughput screening technologies guarantee the rapid acquisition of monoclonal antibodies with specific binding to antigens.
■ Display Technology
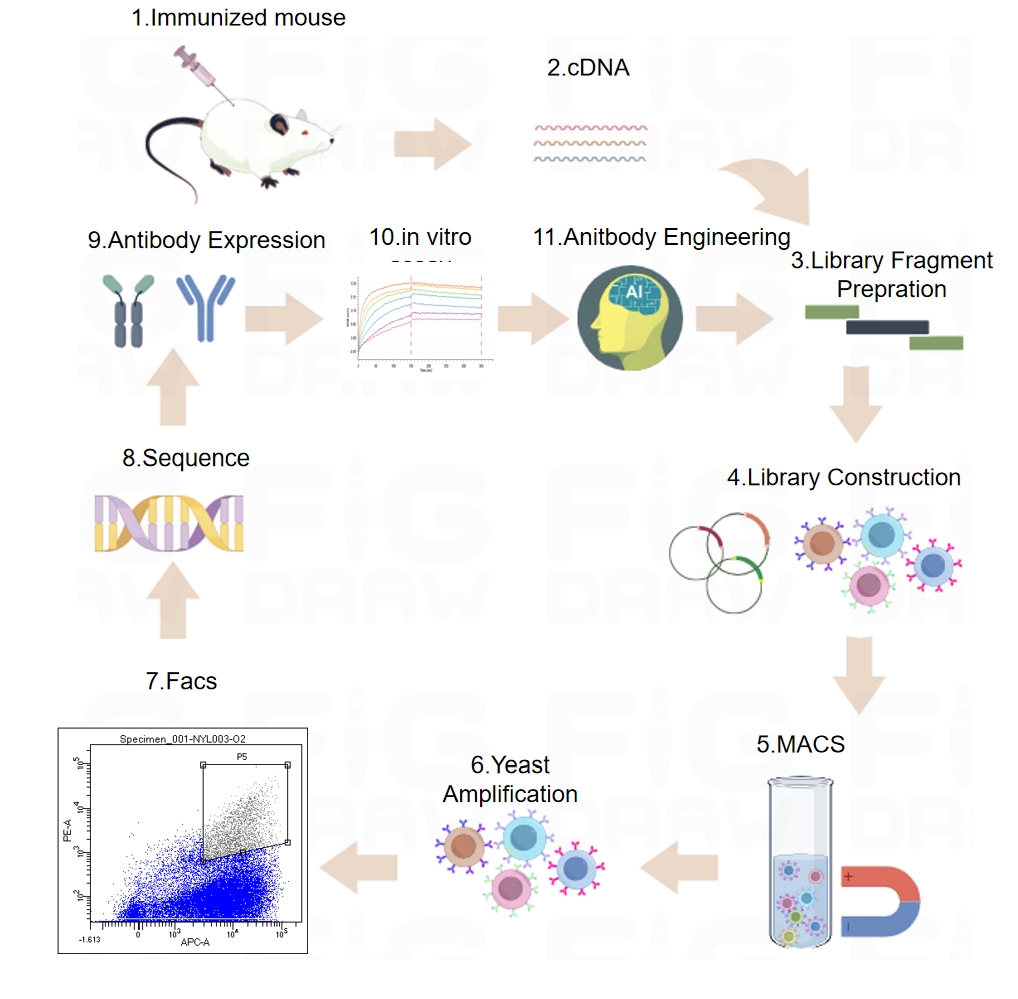
Our Advantages:
Eukaryotic expression system to reduce growth bias.
High-affinity clones are obtained through fluorescence-activated cell sorting (FACS) for visualization.
Easy to perform antibody engineering modifications, such as affinity maturation and Fc engineering.
Epitope selection can be conducted during the antibody screening process.
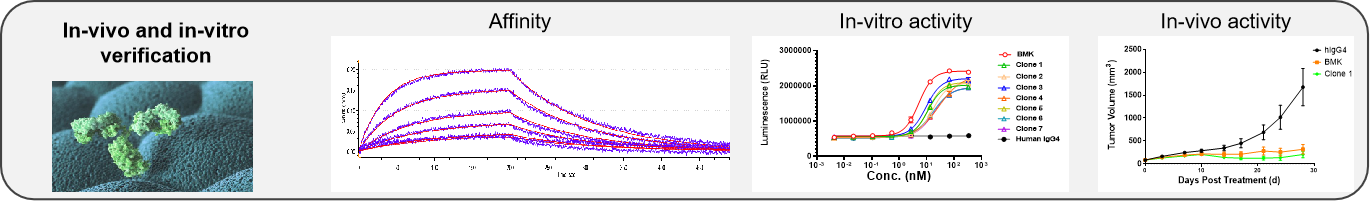
4. In Vivo and In Vitro Activity Evaluation:
Based on the NeoMab® platform and Gempharmatech resources, we can perform in vitro activity evaluations of target molecules according to their mechanisms, including antibody expression and purification, affinity testing, reporter assay, and druggability assessments. Additionally, based on the disease types related to the target, we provide in vivo studies of target molecules, including in vivo efficacy, toxicity, and pharmacokinetics (PK) research for conditions such as tumors, autoimmune diseases, and neurological disorders.
5. Fully Human Antibody Discovery Service Cycle
| Service Content | Antibody Discovery Cycle | Compared to Hybridoma |
| NeoMab® transgenic mouse + Rapid immunization + Single B cell screening | 1 month | 6 months faster than hybridoma |
| NeoMab® transgenic mouse + Rapid immunization + Yeast Display screening | 2 to 2.5 months | 5 months faster than hybridoma |
| NeoMab® transgenic mouse + Rapid immunization + Hybridoma screening | 2.5 to 3 months | 4.5 months faster than hybridoma |
| NeoMab® transgenic mouse + Standard immunization + Single B cell screening | 3.5 months | 3.5 months faster than hybridoma |
| NeoMab® transgenic mouse + Standard immunization + Yeast display screening | 4 to 4.5 months | 3 months faster than hybridoma |
| NeoMab® transgenic mouse + Standard immunization + Hybridoma screening | 4 months | 3 months faster than hybridoma |
| BALB/c or B6 mice + Standard immunization + Hybridoma screening + Antibody humanization | 6 to 7 months | - |

